
The company had recalled the drug, called atorvastatin, in November after particles of glass were found in certain lots.
The original Lipitor, manufactured by Pfizer Inc., has also been the source of some safety concerns.
The U.S. Food and Drug Administration has added warnings to the labels of ...
continue reading...
 A study linked the cholesterol drug Tredaptive to serious side effects that included development of diabetes, gastrointestinal problems, bleeding and infections, according to a Reuters report.
A study linked the cholesterol drug Tredaptive to serious side effects that included development of diabetes, gastrointestinal problems, bleeding and infections, according to a Reuters report. A Massachusetts drug compounder called Pallimed Solutions has voluntarily recalled more than a dozen sterile drugs and compounds following an inspection, CNN reports.
A Massachusetts drug compounder called Pallimed Solutions has voluntarily recalled more than a dozen sterile drugs and compounds following an inspection, CNN reports. A Georgia compounding pharmacy called Clinical Specialties is recalling syringes of the drug Avastin, following reports that they may have caused eye infections.
A Georgia compounding pharmacy called Clinical Specialties is recalling syringes of the drug Avastin, following reports that they may have caused eye infections. A blog post on Forbes deals with Johnson & Johnson’s type 2 diabetes drug Canagliflozin, which is expected to receive FDA approval soon and which the report describes as having “blockbuster potential.”
A blog post on Forbes deals with Johnson & Johnson’s type 2 diabetes drug Canagliflozin, which is expected to receive FDA approval soon and which the report describes as having “blockbuster potential.”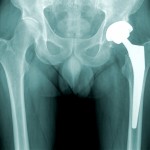 The plaintiff in the second trial over the DePuy Orthopaedics ASR hip implant is seeking punitive damages – indicating “fraud or malice” – as well as compensatory damages, according to Reuters.
The plaintiff in the second trial over the DePuy Orthopaedics ASR hip implant is seeking punitive damages – indicating “fraud or malice” – as well as compensatory damages, according to Reuters.