
A Bloomberg report quotes Blaine Davis, Endo Health Solutions senior vice-president for corporate affairs, as saying: “As a company, we’re very committed to these categories. We are actually investing behind these products.”
Davis said the company, ...
continue reading...
 According to a Bloomberg report, federal regulators have warned Johnson & Johnson’s consumer product division about a number of violations, including the company’s failure to properly review more than 60 medical complaints about a vaginal moisturizer product.
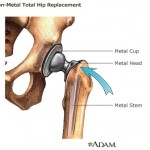
According to a Bloomberg report, federal regulators have warned Johnson & Johnson’s consumer product division about a number of violations, including the company’s failure to properly review more than 60 medical complaints about a vaginal moisturizer product. Some consumer groups are complaining about legislation that recently passed in the U.S. Senate, accusing Congress of passing up an opportunity to keep unsafe medical devices off the market – such as DePuy Orthopaedics
Some consumer groups are complaining about legislation that recently passed in the U.S. Senate, accusing Congress of passing up an opportunity to keep unsafe medical devices off the market – such as DePuy Orthopaedics  According to the Mayo Clinic, hip replacement surgery is generally safe. But as with any surgery, complications can occur. Some complications are serious. But fortunately, most can be treated successfully.
According to the Mayo Clinic, hip replacement surgery is generally safe. But as with any surgery, complications can occur. Some complications are serious. But fortunately, most can be treated successfully.