
According to U.S. News and World Report, the 6-to-4 decision came as a surprise because an FDA briefing document filed Monday in advance of the ruling gave the drug a favorable assessment.
But panel members reportedly said company studies did not provide enough ...
continue reading...
 A study published in The New England Journal of Medicine found more U.S. women had unplanned pregnancies while using short-acting birth control methods such as pills, patches and vaginal rings, compared to long-term reversible contraception methods including intrauterine devices (IUDs), hormone shots and skin implants.
A study published in The New England Journal of Medicine found more U.S. women had unplanned pregnancies while using short-acting birth control methods such as pills, patches and vaginal rings, compared to long-term reversible contraception methods including intrauterine devices (IUDs), hormone shots and skin implants. In a Motley Fool article for MSNBC, Sara Wright recommends against investing in manufacturers of dangerous medical devices that made it onto the market through a regulatory loophole.
In a Motley Fool article for MSNBC, Sara Wright recommends against investing in manufacturers of dangerous medical devices that made it onto the market through a regulatory loophole. Consumers Union, the policy and advocacy arm of Consumer Reports, has placed a full-page print ad in Washington, D.C.-based publication Politico, calling on Congress to close a loophole that allows dangerous devices to make it on the market without clinical testing.
Consumers Union, the policy and advocacy arm of Consumer Reports, has placed a full-page print ad in Washington, D.C.-based publication Politico, calling on Congress to close a loophole that allows dangerous devices to make it on the market without clinical testing.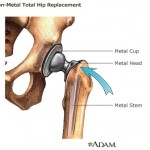 Lawyers representing Pennsylvania and Johnson & Johnson both argued their side before a panel of seven Commonwealth Court justices on Wednesday, in a case involving allegations that Johnson & Johnson defrauded Pennsylvania out of millions of taxpayer dollars.
Lawyers representing Pennsylvania and Johnson & Johnson both argued their side before a panel of seven Commonwealth Court justices on Wednesday, in a case involving allegations that Johnson & Johnson defrauded Pennsylvania out of millions of taxpayer dollars.