
A story in the New York Times says Johnson & Johnson recalled the device, made by subsidiary DePuy Orthopaedics, in 2010. Yet even as the company ...
continue reading...
A story in the New York Times says Johnson & Johnson recalled the device, made by subsidiary DePuy Orthopaedics, in 2010. Yet even as the company ...
continue reading...
Mirena is an intrauterine device that prevents pregnancy for as long as five years. According to the lawsuit that Tricia Prendergast filed in Philadelphia, Bayer doesn’t warn that it can become embedded in the body or cause an ectopic pregnancy. Prendergast claims she required surgery to remove ...
continue reading...
For example, the company was in possession of clinical data showing “extreme” levels of metal ions in patients who received the devices, compared to patients who received a product from a rival company. The all-metal ...
continue reading...
A story in the New York Times notes that the St. Paul-based company has been struggling to deal with problems involving wires that are used to attach the defibrillator to the heart. The wires ...
continue reading...
The story says all-metal hips – which have both a ball and a socket coated with a combination of cobalt and chromium – have been plagued by high early failure rates.
Traditional hip ...
continue reading...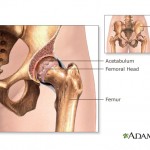
The problem, according to the FDA, is that the implants can shed metal debris when the ball and socket slide against each other during ...
continue reading...California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
