The Consumers Union, the policy and advocacy division of Consumer Reports, has put out a statement applauding the federal Food and Drug Administration’s proposed regulation to establish a “unique identification system” for medical devices marketed in the U.S.
The proposal calls for high-risk devices to carry identification numbers. For most medical devices, the UDI will include a device identifier, which is a unique code tied to a specific device model, and a production identifier, which includes production information for the device.
The ...
continue reading...


 Johnson & Johnson will reportedly pay a settlement in the neighborhood of $2 billion to settle federal charges that the company used illegal tactics in marketing its antipsychotic drug Risperdal.
Johnson & Johnson will reportedly pay a settlement in the neighborhood of $2 billion to settle federal charges that the company used illegal tactics in marketing its antipsychotic drug Risperdal. The federal Food and Drug Administration has unveiled a plan to track high-risk medical devices.
The federal Food and Drug Administration has unveiled a plan to track high-risk medical devices.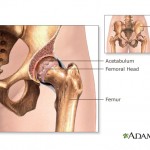 Members of a U.S. Food and Drug Administration panel that recently discussed metal-on-metal artificial hip implants said they wouldn’t recommend that patients get them, although the FDA as a whole has so far stopped short of an official ruling on the devices.
Members of a U.S. Food and Drug Administration panel that recently discussed metal-on-metal artificial hip implants said they wouldn’t recommend that patients get them, although the FDA as a whole has so far stopped short of an official ruling on the devices.