
Although the FDA didn’t detail the number of reports by manufacturer, the Wall Street Journal says, a separate report published in the Archives of Internal Medicine found problems associated with two types of filters ...
continue reading...
Although the FDA didn’t detail the number of reports by manufacturer, the Wall Street Journal says, a separate report published in the Archives of Internal Medicine found problems associated with two types of filters ...
continue reading...
In a press release, CSPI says that Splenda Essentials’ marketing is designed to give the false impression that the sweetener confers health benefits, such as helping its users lose weight and avoid disease.
The lawsuit ...
continue reading...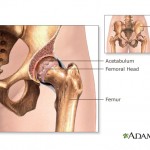
Reuters reports that Synthes issued the recall on July 5, citing the potential for the putty to catch fire if it came in contact with electrosurgical cautery systems during ...
continue reading...
The New York Times quotes Dr. Robert G. Hauser of Abbott Northwestern Hospital in Minneapolis as saying: “There is no need to use this lead until we have more confidence in its performance.”
The devices are wires that connect a patient’s ...
continue reading...
Although the company had already pledged to remove certain chemicals from its baby products by 2013, the new announcement extends the program to its adult products. Johnson & Johnson is the first major consumer products company to make such ...
continue reading...
The report, which cites unnamed sources, says officials with Johnson & Johnson’s DePuy unit recently agreed to settle Nevada residents’ suits over the devices, which were recalled in 2010.
The hip implants in question ...
continue reading...California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
