Although the company had already pledged to remove certain chemicals from its baby products by 2013, the new announcement extends the program to its adult products. Johnson & Johnson is the first major consumer products company to make such a widespread commitment.
The article notes: “Johnson & Johnson’s decision requires the company to navigate a public relations tightrope, by portraying itself as willing to make extensive changes while simultaneously reassuring consumers that its existing products are safe. The endeavor’s success is even more critical because the company has experienced serious recalls and quality lapses in recent years.”
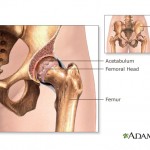
Those recalls include a line of all-metal hip implants that Johnson & Johnson’s DePuy Orthopaedics division was obliged to withdraw in 2010 after nearly half of the patients who received them had to get them replaced within six years. In addition to the high early failure rate, patients had a problem with toxic metal waste breaking off and getting into the bloodstream.
And Johnson & Johnson recently recalled four different types of transvaginal mesh implants, used to treat urinary incontinence and pelvic organ prolapse, after hundreds women who had the devices implanted filed lawsuits alleging that they caused severe pain and injury.
The New York Times says that for years, environmental and consumer groups have pressured Johnson & Johnson and its competitors to remove questionable ingredients from their products.
In 2009, an analysis by the Campaign for Safe Cosmetics found that many products for children contained two substances of particular concern: formaldehyde and 1,4 dioxane. Neither substance is listed on the products because neither is technically an ingredient.
Formaldehyde, which the federal government identifies as a carcinogen, is released over time by common preservatives like quaternium-15 and DMDM hydantoin, which are listed on labels. And 1,4 dioxane, linked to cancer in animal studies, is created during a process commonly used to make other ingredients gentler on the skin.
You should consult with a doctor if you have any ongoing symptoms or health concerns. If you have significant injuries, you should also consult with a lawyer familiar with the DePuy hip implant or transvaginal mesh case to discuss your legal rights.
See the story here:
