Although the company had already pledged to remove certain chemicals from its baby products by 2013, the new announcement extends the program to its adult products. Johnson & Johnson is the first major consumer products company to make such ...
continue reading...
 A drug developed as a cancer treatment proved to be an effective form of birth control for male mice, according to a Bloomberg report.
A drug developed as a cancer treatment proved to be an effective form of birth control for male mice, according to a Bloomberg report. Johnson & Johnson has agreed to pay about $600,000 to resolve three cases in the first settlements of lawsuits related to the company’s
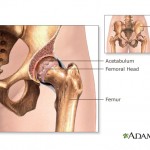
Johnson & Johnson has agreed to pay about $600,000 to resolve three cases in the first settlements of lawsuits related to the company’s 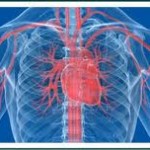 According to a report by ProPublica, medical devices have no unique code that would allow the government to track problems.
According to a report by ProPublica, medical devices have no unique code that would allow the government to track problems. The U.S. Food and Drug administration has ordered St. Jude Medical to do additional studies on patients implanted with medical device components that are blamed in as many as 20 deaths.
The U.S. Food and Drug administration has ordered St. Jude Medical to do additional studies on patients implanted with medical device components that are blamed in as many as 20 deaths.