
The implants, featuring both a head and a socket coated in metal, have been problematic both for their high early failure ...
continue reading...
The implants, featuring both a head and a socket coated in metal, have been problematic both for their high early failure ...
continue reading...
Johnson & Johnson currently markets Xarelto for reducing the risk of blood clots in patients after knee or hip replacement surgery, and reducing the risk of stroke and other blood clots in people with a type of irregular heartbeat. The ...
continue reading...
According to the article, prescription drugs have unique codes that can be used to track problems. But medical devices have no such identifiers and the FDA doesn’t even know how many devices are implanted ...
continue reading...
The federal court decision was in response to allegations that the company used misleading marketing for its antipsychotic drug Risperdal. The jury found that the company failed to properly disclose the drug’s possible side effects, like weight gain, ...
continue reading...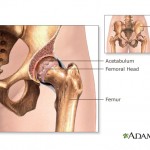
Government prosecutors charge that Johnson & Johnson paid kickbacks to Omnicare, the nation’s largest nursing home pharmacy, to get Omnicare to pick up the antipsychotic medication Risperdal and other Johnson & Johnson drugs.
The federal government ...
continue reading...Lopez McHugh has learned that transvaginal mesh devices, which are causing women extreme pain due to mesh erosion, extrusion, and failure, was approved by the FDA because they were similar another device on the market that has been recalled due to problems.
Yet this approval process is not limited to transvaginal mesh devices. A number of consumer advocacy groups have criticized the U.S. Food and Drug Administration’s 510(k) approval process, ...
continue reading...California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
