Government prosecutors charge that Johnson & Johnson paid kickbacks to Omnicare, the nation’s largest nursing home pharmacy, to get Omnicare to pick up the antipsychotic medication Risperdal and other Johnson & Johnson drugs.
The federal government and a number of states allege that Johnson & Johnson improperly marketed Risperdal for unapproved uses and concealed its risks.
According to the Forbes report, Gorsky served as vice president then president of marketing for Johnson & Johnson’s Janssen unit from 1998 to 2003, which is the time period covered by the government’s complaint. As such, according to the government motion, Gorsky was in a position to know that the misleading marketing was going on.
The U.S. Food and Drug Administration had warned Johnson & Johnson that marketing Risperdal as safe and effective in the elderly was misleading because the drug hadn’t been adequately tested in the population, according to the government motion.
The Forbes report says the government wants to depose Gorsky as part of its litigation, and Johnson & Johnson is fighting the request.
Gorsky is taking over just as Johnson & Johnson deals with some high-profile legal challenges that are casting doubt on the company’s credibility.
The company has instituted more than 30 recalls of medications and medical devices in recent years, and allegedly used deceptive practices in marketing a number of them despite safety concerns.
Johnson & Johnson is still marketing an implant called transvaginal mesh, used to treat women for urinary incontinence and pelvic organ prolapse, despite thousands of reports of adverse events including mesh causing organ perforation, device failure, and debilitating pain.
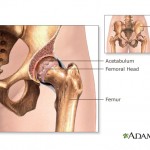
The company also allegedly used deceptive marketing for a type of metal-on-metal hip implant that was recalled in 2010. According to an investigation by British medical journal The Lancet, internal company documents indicate that Johnson & Johnson officials were aware as early as 2005 of the hip implant’s high early failure rate, yet continued to market the device.
You should consult with a doctor if you have any ongoing symptoms or health concerns. If you have significant injuries, you should also consult with a lawyer familiar with the DePuy hip implant or transvaginal mesh case to discuss your legal rights.
See the report here: https://www.forbes.com/sites/erikakelton/2012/04/17/new-jj-ceos-ties-to-fraud-case-show-jj-sees-no-need-for-a-cure/
