
For example, the company was in possession of clinical data showing “extreme” levels of metal ions in patients who received the devices, compared to patients who received a product from a rival company. The all-metal ...
continue reading...
For example, the company was in possession of clinical data showing “extreme” levels of metal ions in patients who received the devices, compared to patients who received a product from a rival company. The all-metal ...
continue reading...
A story in the New York Times notes that the St. Paul-based company has been struggling to deal with problems involving wires that are used to attach the defibrillator to the heart. The wires ...
continue reading...
The story says all-metal hips – which have both a ball and a socket coated with a combination of cobalt and chromium – have been plagued by high early failure rates.
Traditional hip ...
continue reading...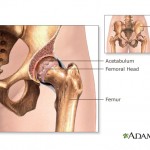
The problem, according to the FDA, is that the implants can shed metal debris when the ball and socket slide against each other during ...
continue reading...
In the first of 1,800 such lawsuits to go to trial, a 47-year-old South Dakota woman named Linda Gross is suing over allegations that the health care giant failed to adequately test the devices or warn patients of their ...
continue reading...
Specifically, the story focuses on an alleged “phantom recall” of painkiller Motrin. According to the story, the U.S. Food and Drug Administration eventually initiated a nationwide recall of more than 88,000 Motrin tablets because of a problem with the way the drug dissolved.
But the ...
continue reading...California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
