
All Eyes on California as $50 Billion Opioid Trial Sets Stage for What’s to Come

First Trial Scheduled in Opioid MDL

U.S. District Judge Daniel Polster had, to this point, sought ...
continue reading...Teva Suspends Zecuity Sales
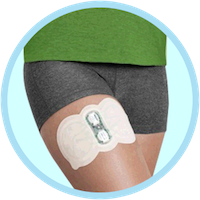
Zecuity’s launch has gone far worse than expected for Teva. The FDA issued a safety alert regarding the migraine relief patch earlier this month after a “large number of patients” experienced burns and scarring on the patch site.
Zecuity was intended ...
continue reading...Migraine Patch May Cause Severe Burns and Scarring

Various prescription pain relievers are available to ...
continue reading...Androgel Manufacturer Drops Plan to Purchase Shire

The Androgel maker was recently in the news over ...
continue reading...