The U.S. Food and Drug Administration has issued what the agency is describing as a “first-of-a-kind guidance” to help medical device manufacturers get through the review process for new devices.
The FDA is issuing the document, titled “Factors To Consider When Making Benefit-Risk Determinations in Medical Device Premarket Approval and De Novo Classifications,” to address problems that have come up recently as a result of the agency’s approval process.
An FDA spokeswoman has been quoted as saying: “The devices center identified several ...
continue reading...

 A post on stock market information Website Seeking Alpha says Johnson & Johnson will likely deal with significant public backlash going forward. An Arkansas jury recently concluded that the company used deceptive tactics to market antipsychotic drug Risperdal, which included hiding or minimizing the drug’s risks. A judge then issued a $1.2 billion fine.
A post on stock market information Website Seeking Alpha says Johnson & Johnson will likely deal with significant public backlash going forward. An Arkansas jury recently concluded that the company used deceptive tactics to market antipsychotic drug Risperdal, which included hiding or minimizing the drug’s risks. A judge then issued a $1.2 billion fine.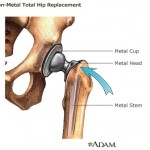 An internal document from the U.S. Food and Drug Administration defends the agency’s rejection of European standards for approving medical devices, according to a report in the Minneapolis-St. Paul Star Tribune.
An internal document from the U.S. Food and Drug Administration defends the agency’s rejection of European standards for approving medical devices, according to a report in the Minneapolis-St. Paul Star Tribune.