The Consumers Union is the policy and advocacy arm of Consumer Reports.
On April 25, the Senate Health, Education, Labor and Pensions Committee was scheduled to mark-up legislation to reauthorize the Food and Drug Administration Safety and Innovation Act on Wednesday. “Mark up” refers to the process by which a committee debates, amends, and rewrites proposed legislation.
“While the legislation includes some improvements over current law, it leaves many significant flaws with the FDA’s current medical device oversight system unaddressed,” the news release states.
Specifically, the legislation fails to address a fast-track review process that allows medical devices to be approved without being safety-tested in humans, based on their alleged similarity to other devices already on the market.
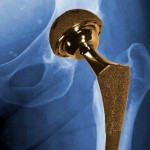
That loophole has resulted in thousands of patients beings injured and even killed by unsafe devices. One example is an all-metal hip implant manufactured by DePuy Orthopaedics, which was recalled in 2010 because of its high early failure rate and tendency to leave toxic metal debris in the bodies of patients.
Transvaginal mesh implants, used to treat urinary incontinence and pelvic organ prolapse, were also approved under the fast-track process. In subsequent years, thousands of patients suffered problems including organ perforation and debilitating pain.
See the news release here: https://www.consumersunion.org/pub/core_health_care/018403.html
