The FDA document amounts to the agency’s defense of its own practices, and describes 12 classes of allegedly “unsafe and ineffective” high-risk medical devices approved for sale in Europe but not the United States.
According to the Star Tribune, the United ...
continue reading...
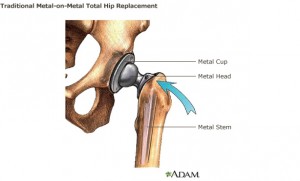
 The American Society of Orthopaedic Surgeons held an expert roundtable on the subject of metal-on-metal hips at the organization’s annual meeting in San Francisco, which took place in February.
The American Society of Orthopaedic Surgeons held an expert roundtable on the subject of metal-on-metal hips at the organization’s annual meeting in San Francisco, which took place in February.