
The agency used “spy software” designed to help employers monitor workers, and captured screen images from the government laptops of scientists as they were being used at work or at home.
Though federal agencies have broad discretion to monitor their employees’ computer use, the F.D.A. program may have crossed legal lines ...
continue reading...
 Stryker Corp. of Kalamazoo, Mich., has announced a voluntary recall of components used in metal hip joints.
Stryker Corp. of Kalamazoo, Mich., has announced a voluntary recall of components used in metal hip joints. Johnson & Johnson will reportedly pay a settlement in the neighborhood of $2 billion to settle federal charges that the company used illegal tactics in marketing its antipsychotic drug Risperdal.
Johnson & Johnson will reportedly pay a settlement in the neighborhood of $2 billion to settle federal charges that the company used illegal tactics in marketing its antipsychotic drug Risperdal.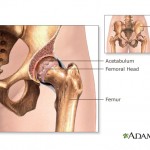 Members of a U.S. Food and Drug Administration panel that recently discussed metal-on-metal artificial hip implants said they wouldn’t recommend that patients get them, although the FDA as a whole has so far stopped short of an official ruling on the devices.
Members of a U.S. Food and Drug Administration panel that recently discussed metal-on-metal artificial hip implants said they wouldn’t recommend that patients get them, although the FDA as a whole has so far stopped short of an official ruling on the devices.