Pennsylvania prosecutors had filed suit against J&J in 2007, trying to recover expenses incurred by the state’s Medicaid program for reimbursing pharmacies over the purchase of Risperdal. Prosecutors alleged that J&J had promoted Risperdal for unapproved – or “off-label” – ...
continue reading...
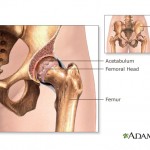 According to a report in U.S. News and World Report, people who have total hip or knee replacement surgery are about 30 times more likely to have a heart attack in the two weeks after the procedure.
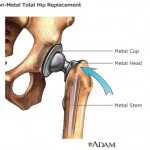
According to a report in U.S. News and World Report, people who have total hip or knee replacement surgery are about 30 times more likely to have a heart attack in the two weeks after the procedure. Johnson & Johnson has set aside $3 billion to deal with the legal fallout from 93,000 all-metal hip implants recalled in 2010, according to a story in the Chicago Tribune. But the healthcare giant may face even more expenses down the line from more problems with a new line of hip implants.
Johnson & Johnson has set aside $3 billion to deal with the legal fallout from 93,000 all-metal hip implants recalled in 2010, according to a story in the Chicago Tribune. But the healthcare giant may face even more expenses down the line from more problems with a new line of hip implants. The Wall Street Journal reports that Johnson & Johnson and federal prosecutors have reached a settlement over allegations that the company illegally marketed its antipsychotic drug Risperdal and other medications.
The Wall Street Journal reports that Johnson & Johnson and federal prosecutors have reached a settlement over allegations that the company illegally marketed its antipsychotic drug Risperdal and other medications. The New York Times reports that the U.S. Food and Drug Administration conducted a wide-ranging surveillance operation against its own scientists.
The New York Times reports that the U.S. Food and Drug Administration conducted a wide-ranging surveillance operation against its own scientists. Stryker Corp. of Kalamazoo, Mich., has announced a voluntary recall of components used in metal hip joints.
Stryker Corp. of Kalamazoo, Mich., has announced a voluntary recall of components used in metal hip joints.