Pennsylvania prosecutors had filed suit against J&J in 2007, trying to recover expenses incurred by the state’s Medicaid program for reimbursing pharmacies over the purchase of Risperdal. Prosecutors alleged that J&J had promoted Risperdal for unapproved – or “off-label” – uses and misrepresented the drug’s safety risks. It’s legal for doctors to prescribe medication for off-label uses that differ from the specific use for which drugs were approved, but illegal for companies to promote drugs for those purposes.
Judges in the Philadelphia Court of Common Pleas threw out the lawsuit in 2010, concluding the state didn’t provide sufficient evidence that J&J’s marketing caused doctors to write off-label prescriptions for Risperdal.
Regardless of the Pennsylvania decision, Johnson & Johnson has reportedly agreed to a settlement of up to $2.2 billion to settle similar charges by federal prosecutors and other states regarding the illegal marketing of Risperdal.
The company is also facing legal difficulties over allegations that it improperly marketed dangerous medical devices.
Up to 10 percent of patients who received transvaginal mesh implants, used to treat urinary incontinence and pelvic organ prolapse, had the devices fail within a year. The most common reported problem is the vaginal mesh eroding and sticking through the walls of the bladder and vagina, causing debilitating pain for patients.
Although Johnson & Johnson’s Ethicon unit recently announced it would stop selling four lines of vaginal mesh devices, the company marketed them despite thousands of reports of device failure, and despite being ordered by the U.S. Food and Drug Administration to stop.
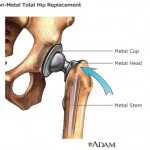
The FDA is also investigating metal-on-metal hip implants manufactured by a subsidiary of Johnson & Johnson in response to thousands of reports that the devices fail prematurely and leave toxic metal debris in patients’ bodies.
The hip implants were recalled in 2010. But an investigation by British medical journal The Lancet found internal company documents indicating that Johnson & Johnson officials were aware as early as 2005 of the device’s high early failure rate, yet continued to market it.
If you’ve received either of these devices, you should consult with a doctor if you have any ongoing symptoms or health concerns. If you have significant injuries, you should also consult with a lawyer familiar with the DePuy hip implant or transvaginal mesh case to discuss your legal rights.
See the story here:
https://online.wsj.com/article/BT-CO-20120726-718781.html
