A letter from the U.S. Food and Drug Administration warns that Johnson & Johnson’s McNeil unit received 227 complaints during a seven-month period about its K-Y Liquidbeads, including 68 unspecified medical complaints that were ...
continue reading...
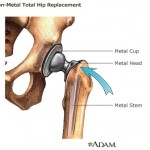
 Some consumer groups are complaining about legislation that recently passed in the U.S. Senate, accusing Congress of passing up an opportunity to keep unsafe medical devices off the market – such as DePuy Orthopaedics
Some consumer groups are complaining about legislation that recently passed in the U.S. Senate, accusing Congress of passing up an opportunity to keep unsafe medical devices off the market – such as DePuy Orthopaedics  According to the Mayo Clinic, hip replacement surgery is generally safe. But as with any surgery, complications can occur. Some complications are serious. But fortunately, most can be treated successfully.
According to the Mayo Clinic, hip replacement surgery is generally safe. But as with any surgery, complications can occur. Some complications are serious. But fortunately, most can be treated successfully. A U.S. Food and Drug Administration advisory panel has voted against approving Johnson & Johnson’s blood thinner Xarelto for prevention of blood clots in people with acute coronary syndrome.
A U.S. Food and Drug Administration advisory panel has voted against approving Johnson & Johnson’s blood thinner Xarelto for prevention of blood clots in people with acute coronary syndrome. Consumers Union, the policy and advocacy arm of Consumer Reports, has placed a full-page print ad in Washington, D.C.-based publication Politico, calling on Congress to close a loophole that allows dangerous devices to make it on the market without clinical testing.
Consumers Union, the policy and advocacy arm of Consumer Reports, has placed a full-page print ad in Washington, D.C.-based publication Politico, calling on Congress to close a loophole that allows dangerous devices to make it on the market without clinical testing.