
A Wall Street Journal report on the policy says that it would require the high-risk devices to carry identification numbers. Jeffrey Shuren, director of the FDA’s medical-device center, described the plan as “a major game-changer” and said the agency plans to ramp up efforts to identify malfunctioning medical devices early.
For a long time, public safety advocates ...
continue reading...
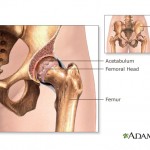 Members of a U.S. Food and Drug Administration panel that recently discussed metal-on-metal artificial hip implants said they wouldn’t recommend that patients get them, although the FDA as a whole has so far stopped short of an official ruling on the devices.
Members of a U.S. Food and Drug Administration panel that recently discussed metal-on-metal artificial hip implants said they wouldn’t recommend that patients get them, although the FDA as a whole has so far stopped short of an official ruling on the devices. Use of metal-on-metal hip implants has been declining in recent years, in response to concerns that they may
Use of metal-on-metal hip implants has been declining in recent years, in response to concerns that they may  A recent report in Scientific American takes a look at a faulty heart device that’s been responsible for at least 20 deaths.
A recent report in Scientific American takes a look at a faulty heart device that’s been responsible for at least 20 deaths.