A letter from the U.S. Food and Drug Administration warns that Johnson & Johnson’s McNeil unit received 227 complaints during a seven-month period about its K-Y Liquidbeads, including 68 unspecified medical complaints that were not adequately evaluated and investigated.
The FDA warning comes at a time when Johnson & Johnson is trying to repair its image after the company instituted more than 30 recalls of medications and medical devices in recent years. Former company CEO Bill Weldon stepped down amid allegations that Johnson & Johnson used deceptive practices in marketing a number of the recalled products despite safety concerns.
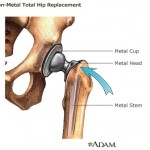
Recalls included all-metal hip implants manufactured by Johnson & Johnson’s DePuy Orthopaedics, which were pulled from the market because of their high early failure rate and complaints of toxic metal debris breaking off and getting into patients’ bodies.
Johnson & Johnson is also facing thousands of lawsuits over allegations that it’s continuing to market a transvaginal mesh implant for treatment of urinary incontinence and pelvic organ prolapse, despite widespread complaints that the device is injuring users.
According to the Bloomberg report, the FDA alleges that Johnson & Johnson failed to maintain adequate complaint files for receiving, reviewing, evaluating and adequately investigating complaints about K-Y Liquibeads.
The FDA also criticized McNeil for failing to submit timely reports about injuries resulting from the use of its Reach dental floss and a separate injury related to use of an O.B. Tampon.
You should consult with a doctor if you have any ongoing symptoms or health concerns from a Johnson & Johnson product. If you have significant injuries, you should also consult with a DePuy hip or transvaginal mesh lawyer to discuss your legal rights.
See the report here:
https://www.nj.com/business/index.ssf/2012/05/johnson_johnsons_mcneil_unit_r.html
