The FDA document amounts to the agency’s defense of its own practices, and describes 12 classes of allegedly “unsafe and ineffective” high-risk medical devices approved for sale in Europe but not the United States.
According to the Star Tribune, the United States generally requires proof that high-risk medical devices benefit patients in “clinically significant” ways, while the European Union generally doesn’t. The latter system can save medical device manufacturers years of expensive clinical trials, and some industry groups and members of Congress have been pushing for changes to make America’s system more similar to Europe’s.
But in recent years, some patient and consumer advocacy groups in the United States have accused the FDA of being too lax in its procedures for approving medical devices.
The independent nonprofit Consumer Reports, for example, has undertaken a national campaign to change the FDA’s procedure for approving medical devices.
Consumer Reports has specifically targeted a fast-track process known as pre-market notification, or 510(k), which allows devices that were never safety tested in humans to be approved based on their supposed similarity to devices already on the market.
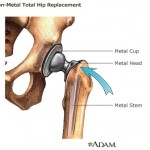
The process led to the approval of metal-on-metal hip implants that Johnson & Johnson subsidiary DePuy recalled in 2010 due to their failure at a higher-than-expected rate, and their tendency to leave toxic metal debris in the bodies of patients.
It also led to the approval of surgical mesh implants, used to treat urinary incontinence and pelvic organ prolapse, which have prompted thousands of lawsuits due to their tendency to fail and cause health problems such as severe, persistent pain and organ perforation.
See the Star Tribune report here: https://www.startribune.com/business/148313295.html?page=all&prepage=1&c=y#continue
