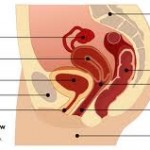
According to Bloomberg, the company informed a federal judge in West Virginia that it intends to stop “commercializing” the implants. Bloomberg quotes a spokesman for Johnson & Johnson’s Ethicon unit as saying that sales of the devices will ...
continue reading...
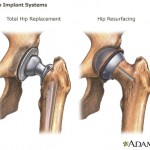 Consumers Union, the policy and advocacy arm of Consumer Reports, has placed a full-page print ad in Washington, D.C.-based publication Politico, calling on Congress to close a loophole that allows dangerous devices to make it on the market without clinical testing.
Consumers Union, the policy and advocacy arm of Consumer Reports, has placed a full-page print ad in Washington, D.C.-based publication Politico, calling on Congress to close a loophole that allows dangerous devices to make it on the market without clinical testing.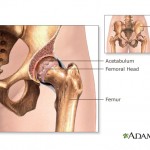 In a motion filed as part of a fraud case against Johnson & Johnson, the federal government alleges that new company CEO Alex Gorsky “was actively involved in matters at issue in this case,” Forbes reports.
In a motion filed as part of a fraud case against Johnson & Johnson, the federal government alleges that new company CEO Alex Gorsky “was actively involved in matters at issue in this case,” Forbes reports.