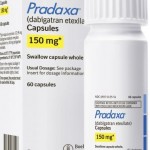
According to the account, she’d been taking anti-coagulant Coumadin for years because of a heart rhythm problem known as atrial fibrillation. Pradaxa was marketed as a therapeutic simplification that, unlike Coumadin, doesn’t require patients to get monthly blood tests.
Boehringer Ingelheim, the manufacturers of ...
continue reading...