As being reported by numerous news agencies, Johnson & Johnson has agreed to settle thousands of pending lawsuits against its subsidiary DePuy Othopaedics arising from its ASR hip implant. Attorneys for the Plaintiffs and Defendants announced the DePuy hip implant settlement agreement during a settlement conference on November 19, 2013 before the Honorable David S. Katz, judge for the United States District Court, Northern District of Ohio, Western Division.
Following the settlement conference, details of the ...
continue reading...
 The National Law Journal is reporting that the first federal trial involving DePuy Orthopaedic Inc.’s metal-on-metal hip implant devices is set to begin on September 9, 2013 in Cleveland, Ohio. This is the first of nearly 8,000 cases currently being coordinated in a multidistrict litigation (MDL) before Judge David Katz.
The National Law Journal is reporting that the first federal trial involving DePuy Orthopaedic Inc.’s metal-on-metal hip implant devices is set to begin on September 9, 2013 in Cleveland, Ohio. This is the first of nearly 8,000 cases currently being coordinated in a multidistrict litigation (MDL) before Judge David Katz.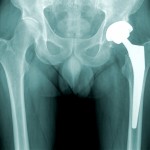 Johnson & Johnson has announced a voluntary recall of millions of oral contraceptive packages, after tests showed that one of the hormones in the pills was being released more slowly than it should.
Johnson & Johnson has announced a voluntary recall of millions of oral contraceptive packages, after tests showed that one of the hormones in the pills was being released more slowly than it should. A story in Canadian news magazine MacLean’s deals with the haphazard nature of medical device regulation, characterizing the problem as a “scandal in the making.”
A story in Canadian news magazine MacLean’s deals with the haphazard nature of medical device regulation, characterizing the problem as a “scandal in the making.” The leading plaintiff trial lawyers association has expressed concerns to the U.S. Food and Drug Administration that a proposed regulatory change might prevent people injured by
The leading plaintiff trial lawyers association has expressed concerns to the U.S. Food and Drug Administration that a proposed regulatory change might prevent people injured by