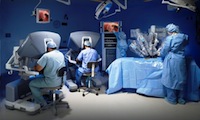
The da Vinci Surgical System allows surgeons to perform operations via remote control, so a physician can maneuver miniaturized surgical instruments in tight areas of the body. The device is often used in procedures where laparoscopic surgery has traditionally been performed, such as cancer surgery, prostatectomies, hysterectomies, and gallbladder removals.
A potential defect in the machine’s surgical scissors may cause injury to other parts of the body, such as cutting an organ that was not the target of surgery. Some surgeons also try to clean surgical instruments inside the body while an operation was in progress, by scraping the instruments together. This could lead to tears or holes in protective covers, in turn causing electrical arcing that could injure patients. Infection is another serious, potentially fatal, risk in either of these situations.
The da Vinci system is used in more than 1,300 hospitals nationwide. From 2000 to 2012, thousands of adverse events related to the da Vinci Surgical System were reported to the FDA. 245 of those incidents involved patient injury, including 71 which resulted in the patient’s death. However, the Johns Hopkins researchers pored through news reports and court documents and identified many adverse events that were never reported to the FDA.
Between 2007 and 2011, the number of procedures in which the da Vinci Surgical System was used grew by 400%. The equipment typically costs a hospital more than $1.5 million, with hundreds of thousands of dollars in annual maintenance costs. To recoup those costs, some medical providers may use it for procedures where it is not necessary or provides no benefit. Intuitive Surgical has come under fire for overly aggressive sales tactics, including promoting the device for procedures not approved by the FDA; pushing for it to be used with patients who are not good candidates for robotic surgery; and attempting to get hospitals to reduce the qualifications necessary for allowing surgeons to use the equipment. One lawsuit has uncovered company e-mails in which they established a policy to persuade physicians to use the da Vinci Surgical System in situations where the surgeon had already decided to use a different method. Some sales reps were told to advise surgeons about which cases were qualified for the da Vinci Surgical System. Other sales reps could actually be found in operating rooms during surgery, advising surgeons about technical difficulties.
Earlier this year, an FDA investigation led to a warning letter in which the FDA criticized the manufacturer’s safety notification process, failure to adequately report device corrections, and failure to inform surgeons of how to clean surgical instruments during procedures or how to transport the equipment between uses.
There have been instances in which an injury caused by the da Vinci Surgical System was not discovered until sometime after the operation. Those persons who believe they may have been injured during one of the types of operation that most commonly involve the da Vinci Surgical System may want to find out if the device was used in their specific procedure. The Lopez McHugh firm is currently reviewing potential da Vinci Surgical System cases. Every state has deadlines for when compensation may be pursued, so you should contact the lawyers at Lopez McHugh without delay.
Contact us now for an initial consultation, free of charge. If we agree to accept your case we will work on a contingent fee basis, which means that we get paid for our services only if there is a recovery by way of settlement or verdict.
