Johnson & Johnson recalled the all-metal implants, manufactured by subsidiary DePuy Orthopaedics, in 2010. At the time, the company said 12 percent fail within five years. Subsequent studies found that nearly half of them failed within six years. Hip implants are supposed to last 15 years or more.
According to the report, thousands of plaintiffs claim they were harmed by toxic cobalt and chromium residue flaking off the devices and getting into surrounding tissue.
Dennis Bobyn, a McGill University professor, testified in Los Angeles at the first trial among 10,000 lawsuits related to the ASR hips.
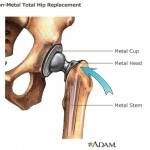
The ASR hips have both a ball and a cup coated with a mix of cobalt and chromium, as opposed to earlier models that incorporate ceramic and plastic. According to Bloomberg, Bobyn testified that the metal cup’s shape, which is less than a half-circle, and the thin wall of the cup contributed to its defective design.
Another problem is that the cup is one piece, rather than two.
Bobyn testified: “Because it has the geometry that it does, it is susceptible to changing shape as it is forced into a patient’s pelvis. If I have a larger object that has to go into a smaller hole, something has to move.”
You should consult with a doctor if you have any ongoing symptoms or health concerns from a DePuy hip implant. If you have significant injuries, you should also consult with a DePuy hip lawyer to discuss your legal rights.
See the story here:
