According to UK newspaper The Daily Telegraph, Miller is chairman of a parliamentary select committee looking into problems with regulation of medical devices in Britain. He’s quoted as saying that if there had been greater transparency in the system, “we wouldn’t have been in this mess in the first place.”
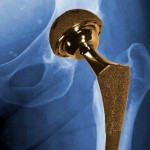
The device in question was based on the design of an all-metal artificial hip from Johnson & Johnson subsidiary DePuy Orthopaedics, which was recalled in 2010 because of its tendency to break down after only a few years. The Telegraph report describes the implant as “toxic,” because of its tendency to shed metal debris in patients’ bodies.
Reporters from both the Telegraph and the British Medical Journal visited a Slovakian firm, posing as representatives of a fictitious Chinese company. They presented plans for an artificial hip based on the DePuy Orthopaedics model.
The firm was tasked with evaluating the device for approval in EU countries, including Britain. Not only did the company officials fail to flag the proposed implant design as problematic, but they expressed their willingness to push for its approval without clinical testing under a regulatory loophole.
The U.S. Food and Drug Administration approved DePuy hip implants without clinical testing under a similar regulatory loophole.
You should consult with a doctor if you have any ongoing symptoms or health concerns from a DePuy hip implant. If you have significant injuries, you should also consult with a DePuy hip lawyer to discuss your legal rights.
See the story here:
