
GBS occurs when the patient’s immune system attacks their nerve cells. It is diagnosed just 3,000 to 6,000 times a year and has been linked as a side effect of other vaccines as well, including ...
continue reading...
GBS occurs when the patient’s immune system attacks their nerve cells. It is diagnosed just 3,000 to 6,000 times a year and has been linked as a side effect of other vaccines as well, including ...
continue reading...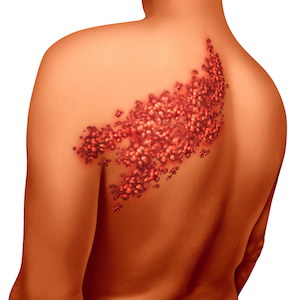
“We understand that this is a challenging situation to manage, and GSK is fully committed to expediting resupply throughout 2018. Going forward, providers and ...
continue reading...
Data was not based solely on efficacy, or effectiveness. Rather, the data also took the vaccine’s safety into consideration. Panel members were “very impressed” with that data on both fronts and – according to Reuters – believe that ...
continue reading...
Such are ...
continue reading...
Zofran is an antiemetic drug used to treat nausea and vomiting in chemotherapy, radiation therapy, and surgery patients. Zofran is also frequently prescribed ...
continue reading...
MDLs are generally used as a tool for increasing the efficiency of of legal proceedings when a number of similar lawsuits are filed. When a large number of lawsuits are filed across a range of ...
continue reading...