Reuters reports that Synthes issued the recall on July 5, citing the potential for the putty to catch fire if it came in contact with electrosurgical cautery systems during ...
continue reading...
Reuters reports that Synthes issued the recall on July 5, citing the potential for the putty to catch fire if it came in contact with electrosurgical cautery systems during ...
continue reading...
The New York Times quotes Dr. Robert G. Hauser of Abbott Northwestern Hospital in Minneapolis as saying: “There is no need to use this lead until we have more confidence in its performance.”
The devices are wires that connect a patient’s ...
continue reading...
Although the company had already pledged to remove certain chemicals from its baby products by 2013, the new announcement extends the program to its adult products. Johnson & Johnson is the first major consumer products company to make such ...
continue reading...
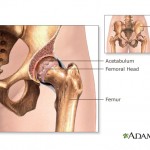
The report, which cites unnamed sources, says officials with Johnson & Johnson’s DePuy unit recently agreed to settle Nevada residents’ suits over the devices, which were recalled in 2010.
The hip implants in question ...
continue reading...
According to the New York Times, the FDA has also recommended that patients who received the Riata defibrillator lead — a wire that connects a defibrillator to a patient’s heart — get X-rays or other imaging to check for problems.
The ...
continue reading...
Sally Gration, 57, underwent hip replacement surgery in February 2006 to treat hip dysplasia, or a malformed hip joint.
Initially, the surgery appeared to be successful. But she began suffering excruciating pain in 2010 – the year ...
continue reading...Categories: Depuy Hip Replacements, Health, Legal Issues, Product Liability, Product News and Recalls, Recall
California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
