
Johnson & Johnson revealed the government investigation in a recent report to the Securities and Exchange Commission.
According to the regulatory filing, the U.S. Attorney’s Office in Massachusetts and the Civil Division of ...
continue reading...
Johnson & Johnson revealed the government investigation in a recent report to the Securities and Exchange Commission.
According to the regulatory filing, the U.S. Attorney’s Office in Massachusetts and the Civil Division of ...
continue reading...
The company is already facing about 10,000 lawsuits connected to another model of all-metal hip implant called the ASR, which was recalled in 2010 over its tendency to break down early and leave toxic metal debris in patients’ bodies.
The Reuters story ...
continue reading...
Studies show that nearly half of the implants, made by Johnson & Johnson subsidiary DePuy Orthopaedics, break down and need replacement after only a few years. They also have a tendency to ...
continue reading...
“Though the company says the evidence will ultimately show that it acted appropriately, it clearly has a lot of explaining to do,” the editorial says.
The model in question ...
continue reading...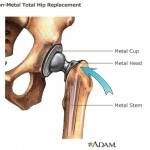
Johnson & Johnson recalled the all-metal implants, manufactured by subsidiary DePuy Orthopaedics, in 2010. At the time, the company said 12 percent fail within five years. Subsequent studies found that nearly half of them ...
continue reading...
“Most companies do everything they can to keep their executives away from court,” the piece states. “A case in California helps to demonstrate why.”
Studies have shown nearly half of the implants break down and ...
continue reading...California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
California
(877) 737-8525 – Toll Free
(949) 737-1501
(949) 737-1504 – Fax
New Jersey
(856) 273-8500
(856) 273-8502 – Fax
Pennsylvania
(877) 703-7070 – Toll Free
(215) 952-6910
(215) 952-6914 – Fax
