Testosterone therapy drugs have been marketed heavily to men with the condition coined Low-T. Men with fatigue or diminished sex drive supposedly do not have enough testosterone, and can reduce these symptoms by taking testosterone replacement drugs. By contrast, some doctors believe that testosterone therapy should be initiated only after multiple very low testosterone readings.
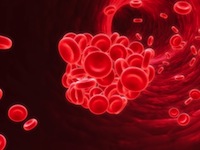
The FDA has already required testosterone products to carry a warning label about the risk of blood clots in the veins due to polycythemia. Polycythemia is a condition that sometimes occurs from testosterone treatment where there is an abnormal increase in the number of red blood cells.

You should consult with a doctor before changing your medication or if you have any health concerns. If you have suffered a heart attack, stroke, or other cardiovascular injury and you were using testosterone therapy at the time, you should contact a testosterone attorney immediately to see if you have a possible case. Call the office of Lopez McHugh, LLP at (877) 737-8525 to schedule a free consultation with a testosterone lawyer today.
