According to Reuters, the problem is a failure to operate properly at extremely high glucose levels.
The report says no patient injuries related to the malfunction have been reported in the United States. The company said there was one report of a serious adverse event outside the United States, but a link to the malfunctioning device had not been determined.
Some recalled items have proven far more harmful for patients.
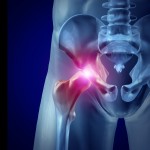
A Los Angeles jury recently awarded $8.3 million in damages to a man who claimed he suffered metal poisoning from an artificial hip implant manufactured by Johnson & Johnson’s DePuy Orthopaedics division, which was recalled in 2010.
That was the first of about 10,000 lawsuits over the hip implant to go to trial. Widespread complaints concern the device’s tendency to break down and need replacement after only a few years, and to leave toxic metal debris in patients’ bodies.
In recent years, the sheer number of Johnson & Johnson recalls has spooked investors. Those recalls included prescription drugs; over-the-counter medicines such as Tylenol, Motrin and Benadryl; and a bone putty used to stop bleeding because it could catch fire during surgery.
According to Reuters, the problems with the blood glucose meters start at extremely high glucose readings of 1024 mg/dl and above. At those levels, the units have failed to provide a warning of dangerously high blood sugar and will shut off, potentially delaying proper treatment.
You should consult with a doctor if you have any ongoing symptoms or health concerns from a DePuy hip implant. If you have significant injuries, you should also consult with a DePuy hip lawyer to discuss your legal rights.
See the story here:
https://www.reuters.com/article/2013/03/25/us-jandj-recall-idUSBRE92O0L220130325
